Strategy
Assessing biological safety is too often a "chicken or egg" game. You test the final form of the device, but then a material or manufacturing process changes and you repeat the tests again. The only way out of the testing cycle is to rely on chemical characterization and expert opinion.
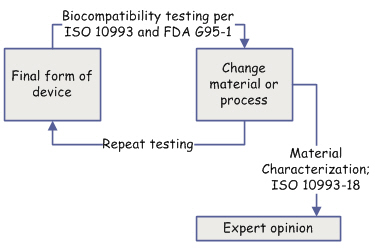
Ideally you'll characterize new materials or process changes before they are committed to design change. But it doesn't always happen that way. The preferred material has ideal performance properties but a questionable test result, you may question the value of an expensive test required by the G95-1 "matrix", or FDA may ask questions about testing included in a 510(k).
Network Staff Associate, Dr. Dan McLain, has experience in material characterization, selection (and exclusion) of biological safety tests, and interpretation of results for implantable, externally communicating, and surface contacting devices.
